
What Can Statins Teach Us About The COVID-19 Vaccines?
Dr. Aseem Malhotra's recent appearance on Joe Rogan will red-pill a lot of people
Note: A summary of this article that is short enough for Twitter can be found here.
Dr. Aseem Malhotra, a renowned cardiologist from England, has done much work over the last decade to expose the cholesterol industry and bring awareness to the statin scam. Because of his scientific literacy and charisma, he has succeeded in building bridges toward controversial ideas with many members of the medical and political establishment. Additionally, he is frequently invited by the mainstream media to provide a dissenting voice on controversial topics—both feats very few have pulled off.
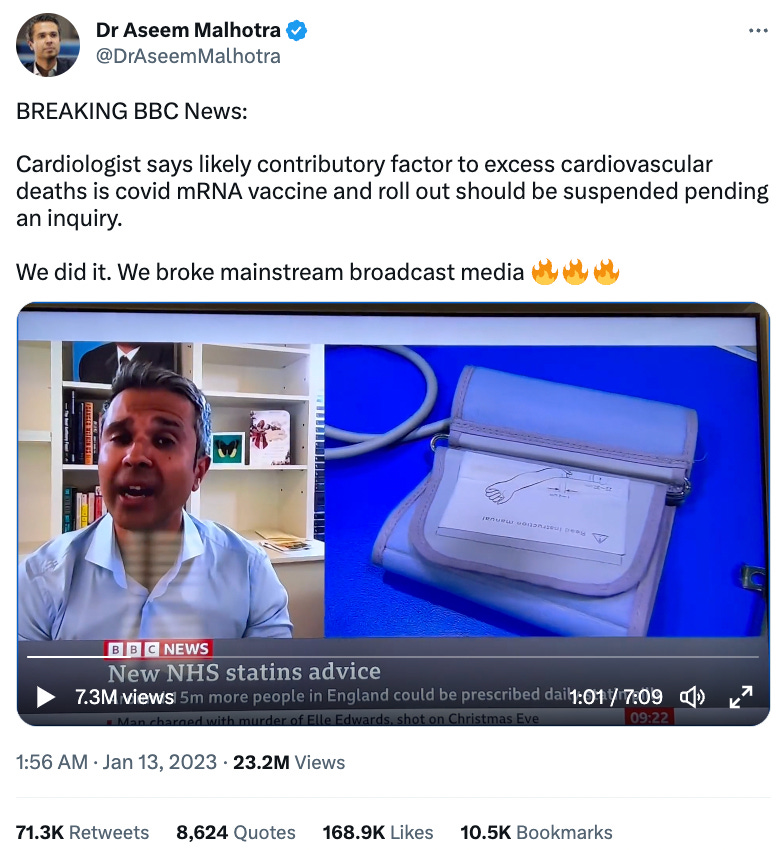
Recently, Dr. Malhotra became red-pilled on the COVID-19 vaccine and began speaking out against them. One of his most notorious accomplishments was breaking the agreed-upon script and smoothly bringing the vaccines up and the deaths they were causing on a national interview for an entirely different subject:
Given that 22 million people saw this broadcast, those supporting the vaccine narrative were not happy about Malhotra’s act of civil disobedience. As had happened previously, when Malhotra publicly spoke out against the cholesterol industry, many over-the-top accusations and cancellation attempts were leveled against him based on the immense harm his “bad” advice would allegedly bring upon the people of England.
Recently, Joe Rogan invited Malhotra on his show and had an excellent interview I believe will go a long way toward both breaking the vaccine narrative and bringing justice to another overlooked problem in medicine—the statins.
Note: many shorter and more shareable clips of the interview have been provided by Spotify and can be viewed on Youtube. These shorter clips will be persuasive when you attempt to share the messages in this article with others.
This interview will red-pill many people, so I hope it will be shared far and wide. It also touches on some points I think are critically important that are the focus of today’s article.
Historical Junction Points
Many of us have realized that the Western Democratic system of government has reached a critical point of unsustainable corruption, and there are essentially three options for where it can go at this point:
•A form of corporate neo-feudalism where most of the impoverished population lives in economic enslavement.
•A complete collapse of the system because the costs of the corruption are unsustainable.
•A reformation of our corrupt institutions and a return to their original state that provided liberty and prosperity to the people rather than the corporate overlords.
Because of how brutal the consequences of the COVID-19 vaccines were to everyone, many, such as Rogan and Malhotra, have begun publicly speaking out against the corruption in our current system so that we can make the third option the outcome we arrive at. These are very unprecedented times, and exactly what we do now will have consequences that ripple out far into the future (this is also why I have invested so much of my time into this Substack).
I Had No Way to Know
One of Dr. Malhotra's admissions in the podcast was that despite dedicating his career to fighting industry corruption and his knowledge they had wholly corrupted the evidence base the practice of medicine is founded upon, it had never even occurred to him there might be something wrong with the COVID-19 vaccines.
Because vaccines were never questioned in his training, it never even occurred to him to question the COVID-19 vaccines. Malhotra just assumed the COVID vaccines were good until he rapidly came face to face with numerous brutal red-pills (such as the inexplicable death of his father) that suggested something was seriously wrong.
To many people, this seems extraordinary and hard to believe. From watching this dynamic for a long time, what instead astounds me was how fast Malhotra switched his position, became willing to acknowledge he was wrong on the issue, and then became willing to take a blowback for publicly stating it.
Very few of us appreciate how many different unquestioned assumptions we need to utilize to function in society and just how much work it is to unravel the fact or fiction behind those assumptions (e.g., doing so can call many other unquestioned beliefs into question). One of the best ways I’ve seen for explaining this concept is to have someone live in a different culture (e.g. China’s) for a few years, as this will force them to realize how many of their fundamental assumptions about the world are not objective truth, but rather simply a product of the culture they grow up in.
Furthermore, the more unquestioned a belief is, the more people become emotionally invested in it. Thus, when evidence is presented questioning any deeply ingrained assumption, people often default to dismissing that evidence.
To some extent, I've gotten around this issue by constructing a belief system designed to encourage the opposite. Specifically, I believe one of my reasons for being alive is to learn as much as I can, and I view learning something I had been sure of, was, in fact, wrong means that I am moving towards a higher level of understanding and thereby fulfilling my life purpose. Empowering belief systems like this one are very helpful, but as the self-help field has demonstrated, most people subconsciously choose to carry disempowering beliefs instead.
Note: one of the wake-up calls I've had from Substack (since I repeatedly fact-check my articles before publication) is how many things I believed to be true were false or impossible to prove and not fit to put into print. My desire to learn where I am wrong is also why I make a point to engage any commenter (provided they are acting in good faith) who asserts something I stated was false.
Because of how complex medicine is, practicing it without carrying countless unexamined beliefs is impossible. As a result, many doctors believe many things about pharmaceuticals (often created by a drug company marketing team) and other medical practices that are simply not true. Even when life forces them to become familiar with the dangers of one medication, they typically do not consider the risks of other medicines (especially ones regularly prescribed by their specialty).
Previously, to explain medical gaslighting, I've argued it primarily results from the fact that doctors (at pharma-funded medical schools) are rarely taught to recognize the adverse events of medications—which matters because most people cannot identify a disease unless they are trained to look for it—and because doctors are human, they do not want to believe they hurt a patient with their medication. However, the broader issue I have not touched on is that practicing medicine requires many unquestioned assumptions; most doctors cannot function if all of those assumptions have to be questioned.
So while medical gaslighting profoundly bothers me, I have a great deal of sympathy for why it occurs and don't believe the issue can be solved by directly attacking doctors (who, in most cases, will just be human and get defensive when it happens). Similarly, I've accepted most drug harms that seem incredibly obvious to me, most doctors will have a great deal of difficulty ever recognizing.
To illustrate the scale of this problem, I would like to share one example that came up for me and was quite humbling.
Since I have been a child, for reasons I still do not understand, I have had a visceral fear and mistrust of pharmaceuticals. Because of this, from a very young age, I talked back to doctors who tried to push drugs on me (which, now that I know what I know, I am profoundly grateful for), I sought out information in the alternative literature about the dangers of many commonly used drugs, and I connected with many who pharmaceuticals had injured.
As a doctor, in contrast to my colleagues who view pharmaceuticals as innocent until proven guilty, I view them as guilty until proven innocent. I've also had a longstanding interest in uncovering the toxicities of medications (e.g., I think a lot about their mechanisms, and I've read Wikipedia's list of every pharmaceutical and tried to classify them by their toxicity). Similarly, I will often go on Facebook and look up if there is a support group for people injured by a pharmaceutical drug and read through it to learn more about that drug's toxicity.
At the same time, I also recognize it's essential not to be close-minded, so I've put a lot of thought into determining which drugs have benefits that outweigh their risks for specific conditions. I even order some of them so I can immediately get them to patients I believe can benefit from them.
With that context, a while back, I had a patient who informed me that she was at her wit's end with Western medicine. I asked for more information, she paused and then told me that a reckless Singulair prescription had ruined her life, that there was a massive community on Facebook of people injured by it (which I later discovered had been around for a long time), and that the FDA even had put out warnings on its side effects.
When she told me this, I paused and went…wow…I had always just assumed that drug was completely safe.
Note: For additional context, one of the most intriguing explanations I have seen of COVID-19 is that the disease is an allergic response to the spike protein. A few colleagues, in turn, have observed spike protein injuries can be treated with allergy medications. Furthermore, they have seen Singulair (an allergy medication) work miracles in more challenging cases.
To share another example of unquestioned medical assumptions, many people who did incredible work to advance early treatment options for COVID-19 have had nasty allegations directed at them for promoting fluvoxamine as an early treatment option. Those allegations were based upon SSRI antidepressants frequently being extremely dangerous (which is true), that they could not possibly have any benefit, and that fluvoxamine has harmed COVID-19 patients who took them.
I do not agree with this rational for the attacks against the fluvoxamine advocates for three key reasons:
•Many people who promoted fluvoxamine, I know with certainty, did this because they thought it was the best thing they could do to help COVID-19 patients.
•At the time fluvoxamine was looked at, very few treatment options existed for COVID-19, and data showed brief fluvoxamine therapy worked. Many people who initially endorsed it have since dialed their support back (e.g., they've made it a third-line agent in their treatment protocols) as they've found other therapies, they've seen it does not work as well on the newer COVID variants and some of their patients have had difficulty tolerating fluvoxamine treatment.
•Even though the harms of antidepressants are incredibly obvious and affect immense numbers of people, even now, decades since they entered the market, very few people in the medical field are aware of their dangers. Because of this, it was not realistic for the initial advocates of the fluvoxamine to have known how dangerous it was.
Cholesterol and Heart Disease
Frequently, when an industry harms many people, it will create a scapegoat to get out of trouble. Once this happens, a variety of other sectors which also benefit from that scapegoat existing will jump on the bandwagon. Before long, a false belief that harms society becomes an unquestionable dogma that becomes very difficult to overturn because many corrupt parties have a vested interest in maintaining the lie.
For example, various easily addressable factors (which often exist because they benefit an industry) are responsible for the chronic diseases we face in society and our vulnerability to infectious diseases (e.g., the obese and diabetics were much more likely to catch COVID-19). By saying all disease results from insufficient vaccination, it gets all those destructive industries off the hook and creates a huge market for selling vaccines and treatments for these illnesses. Thus, since there are so many vested interests behind the vaccine paradigm, it is very difficult to overturn.
In the 1960s and 1970s, a debate emerged over what caused heart disease. On one side, John Yudkin argued that the sugar being added to our food by the processed food industry was the chief culprit (e.g., in his seminal book, he stated, "I hope that when you have read this book, I shall have convinced you that sugar is really dangerous.").
On the other side, Ancel Keys (who attacked Yudkin's work) argued that it was due to saturated fat and cholesterol. Ancel Keys won, Yudkin's work was largely dismissed, and Keys became nutritional dogma. It gradually became recognized that Ancel Keys did not accurately report the data he used to substantiate his arguments. Fifty years after the initial debate, one of the most prestigious medical journals in the world published internal sugar industry documents. They showed the sugar industry had used bribes to make scientists place the blame for heart disease on fat so Yudkin's work would not threaten the sugar industry.
It is now generally accepted that Yudkin was entirely right and had we listened to him, an immense amount of suffering could have been prevented. Despite this, the cholesterol hypothesis of heart disease persists (despite very little evidence supporting it and a significant amount refuting it), and we still provide diabetics with disastrous dietary (eat carbs and no fats) advice that originated from the work of Keys.
Note: although sugar (and fructose) is one of the primary causes of heart disease, other primary causes, such as smoking and lead exposure, also exist. Additionally, research has actually shown cholesterol greatly increases longevity. To quote one article from the Lancet:
During 10 years of follow-up from Dec 1, 1986, to Oct 1, 1996, a total of 642 participants died. Each 1 mmol/L increase in total cholesterol corresponded to a 15% decrease in mortality (risk ratio 0–85 [95% Cl 0·79–0·91]).
Statin Marketing
For decades, researchers have looked for ways to lower cholesterol levels reliably. Once statins (the first drugs which could reliably do this) were discovered, the cholesterol hypothesis took off, and reasons were created to create more and more urgency for lowering cholesterol levels. This has gone to the point prominent doctors have called for statins to be added to the water supply, a degree of fanaticism not that different from what we saw from many of the advocates for mass COVID vaccination.
Since the rationale for statin usage is based on a lie, the benefits of statins are almost nonexistent. Similarly, since cholesterol is essential for life, many issues result from eliminating it. Nonetheless, statin sales are now over 15 billions dollars a year, and hundreds of millions of people have been placed on them.
This raises an interesting question. How on earth is this possible?
The answer is of course, very good marketing funded by an incredibly lucrative product.
Risks and Benefits
Before COVID vaccines, I considered statin medications to have one of the worst benefit-to-harm ratios of any drug on the market. To put this into context, based on clinical trials already biased in favor of the drugs and selecting those most likely to benefit from statin usage, it was found on average, taking a statin for five years would increase your expected lifespan by three days.
Conversely, beyond the medication costs (which many struggle to afford), it can be expected that around 20% of those receiving a statin will experience side effects from the drugs. 20% represents the best estimate Malhotra has been able to make from studying all the available data).
Since a 20% adverse event rate would destroy their sales, the statin industry has used a variety of tricks to argue the adverse event rate is under 1%. For example, in their trials, they will often pre-test statins on participants, remove those with bad reactions, and then make up excuses like the individuals (who had volunteered to be test subjects) not wanting to take their medication to justify removing them from the trials.
Two of the most compelling pieces of evidence on this subject Malhotra cited to Rogan were as follows:
•A large study in the USA showed within a year of being prescribed statins, 75% will stop taking them, and when you ask why, 62% of the 75% who stopped will say it is because of statin side effects.
•The same leading statin "experts" at Oxford who claim statin side effects happen to less than 1% of recipients determined statin side effects were more likely to occur in genetically susceptible individuals (which is true—typically, it applies to those who experience more significant cholesterol reductions from regular statin doses). This is important because (until they were publicly shamed for it) in the marketing those same experts used to sell their test, they said 29% of all statin users were likely to experience side effects.
Unfortunately, the statins have since lost their distinction for having the worst risk-reward ratio of any drug on the market.
When the initial Pfizer vaccine study was published in the Lancet, all of my colleagues were captivated by the claim the vaccine was "95% effective," and they all repeated the mantra, "well, we had hoped the vaccine would work, but we never imagined it would be this effective." I took a look at Pfizer's study and noticed that:
You had to vaccinate 119 people to prevent one minor case of COVID-19 (e.g., a sore throat + a positive test), 2711 people to prevent one "severe" case of COVID-19 ("severe" never being defined), and since no deaths were prevented in the trial, well over 21,720 people needed to be vaccinated (21,720 is the total number who were vaccinated in the trial) to prevent a single death from COVID-19. Additionally, the vaccine's ability to prevent transmission was never assessed, and based on my knowledge of the vaccine design (which was unlikely to prevent transmission), it was unlikely to do so.
Conversely, a high rate of side effects was reported in the trial (e.g., 59% experienced fatigue), which dwarfed the benefits attributed to the vaccine preventing minor cases of COVID-19.
Knowing that most trials are fraudulent (so the benefits were likely exaggerated, and many of the side effects were unreported), I could only wonder how bad the risk-reward ratio of the vaccine was if something that bad was being publicly presented. Also, remember that this was the best time for the vaccine to "work" as most of the population did not yet have a natural immunity to COVID-19 (thereby invaliding any benefit of the vaccine), and the virus had not yet mutated to a strain the vaccine did not cover.
Subsequently, we learned that:
•Like many other industry trials, many severe adverse events in Pfizer's trials were never reported.
•The trial was not blinded, and Pfizer deliberately did not test vaccinated individuals for COVID-19 (thereby erasing any benefit the vaccine did have).
•At six months of follow-up, in both Pfizer's and Moderna's trials, more vaccinated than unvaccinated individuals died.
•In a peer-reviewed reanalysis of Pfizer and Moderna's trials by some of the top academics in the world, it was determined that the trial data showed one was more likely to suffer a severe adverse event from the vaccine than a hospitalization from COVID-19.
Since the vaccine trial's data was heavily biased in favor of the vaccines, real-world data demonstrated the bar actually could go far lower. In Malhotra's interview, he cited data made available by England's health service (which Malhotra noted, due to the healthy user bias, was likely still overestimating the benefits of the vaccines). It found that depending on one's age, between 2500 people (for those over 70) to well over 100,000 people (for those under 30) would need to be vaccinated to prevent a single hospitalization. This benefit is in trying to end poverty by having the government pay for everyone to get a few free lottery tickets.
Conversely, as we all know, the harms of the vaccine are orders of magnitude greater than the benefits. Although no precise figures will likely ever exist, I will cite two of the better overall estimates of harm available (remember that these are not assessing for specific vaccine side effects like myocarditis).
Ed Dowd's team, using a fairly conservative methodology (so there was no risk of overestimating the harms), found that of those who received the vaccine:
•82% had no significant adverse effect
•18% injured (26.6 million total Americans)
•0.93% were injured and disabled (1.36 million total Americans)
•0.05-0.1%, injured and dead (300 thousand total Americans).
A poll by Rasmussen Reports found that:
•7% of those vaccinated believe they suffered a significant side effect
•34% of those vaccinated believe they suffered a minor side effect
•56% believe that they had no side effect
•4% were not sure if they suffered a side effect.
Given that I frequently observe side effects the vaccinated individual (including doctors) did not recognize until I point them out, the Rasmussen poll is likely underestimating the rate of vaccine injury.
This effectively means that in many cases, you are over 10,000 times as likely to be significantly harmed by the vaccine as you are to benefit from it, preventing a COVID hospitalization.
The saddest thing about this is that many have tried to nonsensically argue the massive rise in deaths we've seen after the vaccines and vaccine mandates are actually due to patients not seeing their doctors and taking as many "life-saving" statins.
Pushing Poisons
When you look at how the market for statins has been created, there are an incredible number of parallels to what we have seen during COVID-19. I would argue this is because the statins played a pivotal role in creating the playbook that was reused throughout the pandemic. Similarly, many of those who dissented from the narrative could immediately recognize what was happening because they had already gone through it with previous pharmaceuticals (e.g., what was done with the SSRIs).
Let’s look at a few of the ways unjustifiable pharmaceuticals are marked on everyone:
Buying Scientific Research
Many academic experts have been bought off to produce studies arguing for more and more statin usage. Simultaneously, many medical journals have also been bought out and will only publish studies that favor the use of statins. This results in an overwhelming amount of evidence in favor of the drugs, despite their benefits being almost non-existent and their harms quite frequent.
As you may have noticed, this is the identical situation we face with the COVID-19 vaccines. Despite widespread evidence of harm, it is nearly impossible to get studies critical of the COVID-19 vaccines published. When they nonetheless are, they are then retracted for spurious and nonsensical reasons.
One of the most notorious examples of corrupt statin research was this study that concluded the side effects individuals experience from statins were due to the nocebo effect (the belief statins could cause harm) rather than the statins themselves. There were so many issues with this study, but all the statin apologists jumped on it, and I repeatedly saw cases where someone developed statin symptoms after a statin, but when they talked to their doctor, to share one example I witnessed:
This was not due to the statin…in my career, I’ve never seen a single case of statin-induced muscle pain [when I heard the doctor say this, I immediately knew why]…they even recently did a study that showed those “side effects” are just due to the nocebo effect…you may not under any circumstances stop the statin because if you do, you will get a heart attack and die.
Concealing Scientific Data
Previously, one of the most egregious offenders in this regard was the statin manufacturers who have deliberately withheld their data from the public for decades. A corrupt Oxford academic consortium, the Cholesterol Treatment Trialists' (CTT) Collaboration, has access to that data and has published numerous pro-industry analyses of it but, despite continual outside requests, has refused ever to make this data available for outside scrutiny. Given the significant evidence demonstrating that statins are both ineffective and harmful, many more honest academics have attempted to independently obtain this critical data from regulators.
Note: this is the same group that denied adverse events existed from statins while it simultaneously sold the genetic test for your risk of an adverse event to a statin.
Almost all of the COVID-19 vaccine data likewise was never made available to the public (although the companies have suggested it may be made available a few years from now); instead, we received highly curated publications in prestigious medical journals. Since the vaccines entered the market, countless red flags on their safety and efficacy have emerged in large datasets. However, in many cases, that data has only been available because it was leaked by whistleblowers or obtained by court order, and as the recent events in Israel showed (Israel agreed to be Pfizer's laboratory to test their vaccines and many global vaccine policies were crafted from the Israeli data), much of the incriminating data against this program was deliberately concealed by governments around the world.
In both cases, it is almost impossible to believe we are being forced to take something based on a "scientific" consensus that it is incredibly safe and effective, yet we can't even see the data that consensus is based upon.
Guideline Committees
As time has gone forward, a great deal of effort has been made to transform the practice of medicine from doctors independently utilizing their best judgment on how to treat patients to doctors following treatment algorithms that committees of experts create. This arrangement creates a creative way to skirt the law since these committees do not require a legislative process to be enacted. In turn, a few times, they have been sued for the absurd guidelines they put forward.
In each case (e.g., the recent one against the FDA for it preventing ivermectin from being used to treat COVID-19), those promoting the guidelines successfully argue their guidelines are only ‘suggestions’ and thus cannot be legally challenged. This is important to remember since, in one ruling against Lyme patient advocates, the federal judge specified that guidelines are voluntary (which means they cannot be treated as law). Nonetheless, once these “voluntary” guidelines are created (and hence cannot be challenged through any legal process), everyone treats them as law.
Given the importance of these “guidelines,” it should then raise the question of why they always seem to arrive at conclusions that favor industry. As you might imagine, the formula is quite simple—almost everyone who ends up on those committees coincidently also takes money from those who are financially invested in the outcomes of their guidelines.
In the case of statins, a relatively simple pattern has emerged. As time goes forward, “research” keeps appearing that suggests more people need to be placed on statins. The experts on the guideline panels then conclude that even more people need to be given statins, and clinical practice guidelines are published requiring this, which doctors are sanctioned for failing to follow (e.g., Medicare gives them less money).
One of the best examples of this was shared by Dr. Malcolm Kendrick in chapter 7 of Doctoring Data:
The National Cholesterol Education Programme (NCEP) has been tasked by the NIH to develop [legally enforceable] guidelines for treating cholesterol levels. Excluding the chair (who was by law prohibited from having financial conflicts of interest), the other 8 members on average were on the payroll of 6 statin manufacturers. In 2004, NCEP reviewed 5 large statin trials and recommended: “Aggressive LDL lowering for high-risk patients [primary prevention] with lifestyle changes and statins.” [these recommendations in turn were adopted around the world]
In 2005 a Canadian division of the Cochrane Collaboration reviewed 5 large statin trials (3 were the same as NCEP’s, while the other 2 had also reached a positive conclusion for statin therapy). That assessment instead concluded: “Statins have not been shown to provide an overall health benefit in primary prevention trials.”
In addition to doctors being forced to follow these guidelines, patients often are too. Doctors often retaliate against patients who do not take statins (similar to how many unvaccinated patients were denied essential medical care during COVID-19). Employers sometimes require cholesterol numbers to meet a certain threshold for employment (although they never did anything on the scale of the COVID vaccine mandates placed on workers around America). Similarly, life insurance policies often penalize those with "unsafe" cholesterol numbers.
Corrupt guideline committees also played a pivotal role in shaping the disaster we saw unfold over the last two years. The most clearcut example occurred with the federal guideline panel chosen to determine the appropriate treatments for COVID-19 (whose decisions, in turn, were effectively law for the treatment of COVID-19 across America).
Without any outside accountability, Fauci (who was heavily invested in remdesivir) appointed the entire panel. His panel was composed of his longtime friends, and more importantly, for almost every member, it could be proven that remdesivir's manufacturer was paying them off. Unsurprisingly, the panel continually voted for remdesivir (an ineffective and harmful drug) to be the standard of care.
More importantly, it continually voted against using any off-patent drug to treat COVID-19, despite the evidence for these drugs being dramatically stronger than that for remdesivir. Because of this panel, despite many treatments existing for COVID-19, none was ever made available to the American people. As a result, the pandemic was allowed to continue for years rather than the few months that would have been sufficient to end it.
Similarly, once the COVID-19 vaccines hit the market, the FDA and CDC guideline committees continually voted to approve the vaccines and "recommend" them to the American population. This was done despite the large public protest and existing evidence making many of those justifications completely unjustifiable (e.g., for children with a 0% risk of dying from COVID-19 and a significant risk of being harmed by the vaccines).
Since the goal is always to increase sales, once one market is established, guideline committees are repeatedly called upon to increase the number of eligible customers. In the same way, we saw this done by forcing the vaccine upon more and more demographics, the cholesterol numbers deemed necessary to start statin treatment are continually lowered, thereby making more and more of the population be heckled to start statins.
One of the most comical examples of this was the calculator made by the American College of Cardiology to determine your risk of developing a heart attack or stroke in the next ten years. I lost track of how many doctors I saw proudly punch their patient's numbers into it and then inform them that they were at high risk of a stroke or heart attack and urgently needed to start a statin. As I watched this, I could not help but notice how many patients met this threshold and that the calculator's results were incongruent with the much lower rates of heart attacks and strokes I saw occurring around me.
I was thus delighted to learn Kaiser completed an extensive study that determined the American College of Cardiology's calculator overestimated the rate of these events by 600%. Sadly, like all the evidence showing the COVID-19 vaccines only cause harm, that study did not affect the usage of the calculator, and nearly ten years later, I still see the calculator frequently utilized to push statins on patients (which may be partly due to it still being used within medical board examination questions).
Statin Gaslighting
Is it any wonder doctors, when hearing these patient complaints of tiredness, weakness, wobbly-kneed with burning pain and numbness, poor coordination and terrible memory, respond with a predictable, "You are over fifty now and have to expect these kinds of things.
I’ve made peace with the fact many useless drugs are on the market, and now I only focus my energy on protesting medications that harm patients.
Ever since the statins hit the market, colleagues and I began observing case after case of individuals who lost sensation in their body, developed muscle pains, or had cognitive decline set in once they started the statin, which immediately resolved once they stopped the statin. Simultaneously, we also noticed that whenever we pointed this out to their doctor, the doctor would become extremely hostile, and then both insist that the statin could not be causing the symptom and that even if it was, the patient needed not to stop using it because otherwise they would get a heart attack and die.
The thing that finally really made me get how impressive the marketing for these drugs had been was the recurring battle I would have with relatives. In each case, I would take them off a statin and provide a strong argument with data supporting why they should not be on the drug. At some point later, they would go to their doctor and inform them that their relative, who was a doctor, had taken them off the statin.
Their doctor (often a cardiologist), in turn, would tell my relative I was incredibly ignorant, insist they knew the data much better than I did, say I was endangering my relative’s health, and promptly restart the statin, to which my relative dutifully complied. In many cases, I would provide the cardiologist with literature supporting my argument. In each case, they would make an excuse not to read it while simultaneously asserting they knew all the data and that I, not being a cardiologist, was unqualified to have an opinion on this subject. This made me appreciate just how challenging a situation patients (without access to the resources my relatives had) were in.
If you take this story and replace “statin” with COVID-19 vaccines, you will see it is essentially what everyone has experienced over the last two years with the vaccines. I suspect this is because, before the COVID-19 vaccines, statins were one of the most profitable medical franchises and, thus amongst the medications most aggressively pushed on patients.
Note: two adverse event reporting systems exist for adverse reactions to pharmaceuticals, MedWatch and FAERS. Like VAERS, they suffer from severe underreporting (it is estimated only 1-10% of adverse events are reported to them). The author in the next section was able to find hundreds to thousands of reports for many of the statin injuries in MedWatch that watched what he had personally observed. However, despite these reports existing, nothing has been done with them, and there is almost no knowledge within the medical community that these adverse events exist.
Statin Damage Crisis
“Many statin victims say that abruptly, almost in the blink of an eye, they have become old people.”
Duane Graveline MD was started on a statin and soon after developed global amnesia (which is really scary). He decided to stop the statin and recovered.
When I suggested, on the basis of my 23 years as a family doctor, that perhaps my new medicine was the cause of my amnesia, the neurologist replied, almost scoffingly, that "Statins do not do that." He and many other physicians and pharmacists were adamant that this does not occur.”
Eventually, he was persuaded to try again.
The year passed uneventfully and soon it was time for my next astronaut physical. NASA doctors joined the chorus I had come to expect from physicians and pharmacists during the preceding year, that statin drugs did not do this and at their bidding I reluctantly restarted Lipitor at one-half the previous dose. Six weeks later I again descended into the black pit of amnesia, this time for twelve hours and with a retrograde loss of memory back to my high school days.
Later he discovered:
Perhaps stockholder loyalty explains why Pfizer management knew over a decade ago, during the first human use trial of Lipitor, of the cognitive impact to come when Lipitor was released to the public. Of their 2,503 patients tested with Lipitor, seven experienced transient global amnesia attacks and four others experienced other forms of severe memory disturbances, for a total of 11 cases out of 2,503 test patients. This is a ratio of 4.4 cases of severe cognitive loss to result from every 1000 patients that took the drug. Not one word of warning of this was transmitted to the thousands of physicians who soon would be dispensing the drug.
Because of this and other debilitating long-term complications (e.g., previously an extremely fit individual, he developed chronic exhaustion), Graveline became an expert on statin injuries and, in 2014, wrote The Statin Damage Crisis. To my knowledge, it is the most detailed book that has been written on the harms of these drugs. This section, in turn, is a summary of part of it.
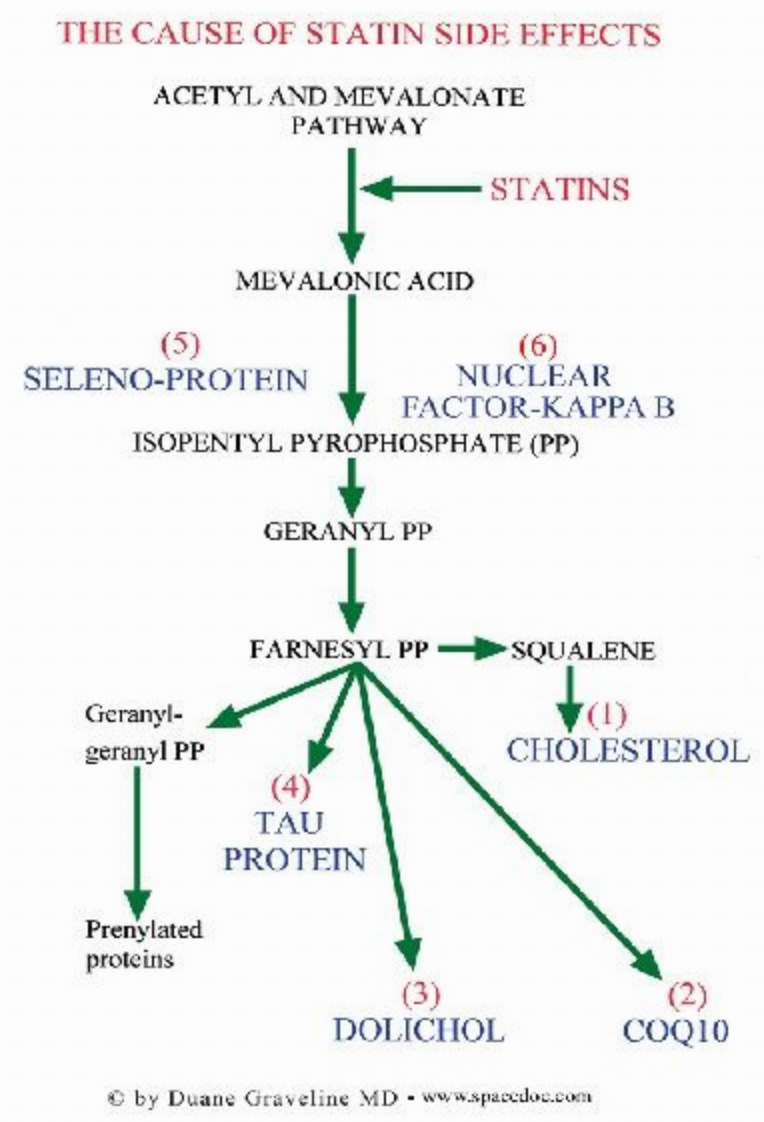
Statin work by inhibiting an easy to target enzyme that is necessary for the production of cholesterol. Unfortunately, doing that affects a variety of vital other physiologic processes, too, which most physicians have almost no knowledge of. This biochemistry is important to understand because it explains most of the adverse effects of the statins.
Note: many of the side effects here overlap with those observed in COVID-19 recipients and some of them will be further discussed below.
Cholesterol
Cholesterol has a few different essential functions in the body. These include:
•It is the precursor to hormones throughout the body.
•The brain’s synapses (which, amongst other things form memories) require cholesterol to function. Since cholesterol is too big to enter the brain, glial cells (support cells of the nervous system) synthesize it within the brain. Statins, unfortunately, inhibit glial cell production of cholesterol.
A variety of cognitive effects such as amnesia, forgetfulness, confusion, disorientation, and increased senility, in turn, have frequently been observed with statins.
Their patient’s rapid descent into dementia after a statin is started is much too often written off by their doctor as senile brain changes or beginning Alzheimer's when the real culprit is their statin drug.
Note: one of the sadder side effects we have frequently observed from the COVID vaccines has been a rapid cognitive decline in the elderly (who cannot often advocate for themselves). When this happens, it is always assumed to be due to “their age” and ignored.
Any time a severe side effect is associated with a pharmaceutical (e.g., sudden death after a COVID vaccine or complete amnesia after a statin), it means that less severe versions of that side effect also occur at a more frequent rate. In addition to the moderate forms of cognitive impairment discussed above, Graveline found a study that showed minor cognitive impairment could be detected in 100% of statins if sufficiently sensitive testing was done.
In addition to cognitive impairment, numerous studies have found a significant association between low or lowered cholesterol levels and violence.
Finally, one of the most concerning side effects of statins is their tendency to cause ALS (a truly horrible rare disease—curiously also seen in association with the COVID-19 vaccines). This correlation is further supported by many reports of statin ALS improving once the statin is stopped.
Unfortunately, while statin cognitive decline frequently improves when the statin is stopped, in many cases, it instead persists.
CoQ10
CoQ10 is an essential nutrient both the mitochondria (which power the human body) and the stability of our cell walls depend upon. CoQ10 deficiency caused by statins is generally considered the most common cause of their side effects. This is really sad because those side effects could have been prevented if CoQ10 had been given with the statin.
Unfortunately, this is unlikely ever to happen, as doing so would be equivalent to an admission statins could cause harm. The best parallel I know to this is that the primary cause of childhood vaccine toxicity is too many vaccines being given too close together for a child's developing circulatory system, which becomes overwhelmed and suffers debilitating interruptions in the blood supply. Most of the harm can be avoided if vaccines are spaced apart and given later in a child's life.
Holistic pediatricians who practice this approach so parents have the safest option to get their child the vaccines required for school are typically targeted (in some cases losing their licenses—often with spurious justifications such as "they aren't following the CDC guidelines"). I believe this occurs because their approach is equivalent to admitting vaccines are not 100% safe, and the industry cannot afford for it and its implied message to catch on.
Some of the common energy-related side effects of statin CoQ10 deficiency include:
•Mitochondrial damage
•Lack of Energy
•Chronic Fatigue Syndrome
•Congestive Heart Failure and Fluid Retention
•Shortness of Breath
•Gout
Some of the side effects of statin CoQ10 deficiency weakening cell wall integrity include:
•Hepatitis (interestingly, Graveline noted that the enzyme threshold needed to diagnose statin-induced liver damage was significantly raised after this issue began being commonly reported following statin usage).
•Pancreatitis
•Rhabdomyolysis (rapid breakdown of skeletal muscle tissue)
•Tendon and ligament inflammation and rupture (note: as this side effect is commonly reported with fluoroquinolone antibiotics, which are known to damage the mitochondria, it may be primarily due to statins' effect on the mitochondria. My colleagues and I also know a few people this happened to due to a COVID-19 vaccination).
Two of the most common CoQ10 side effects of statins are myopathy (muscle pain, tiredness, weakness, and cramps) and peripheral neuropathy (numbness, tingling, or burning sensations, particularly in hands and feet).
Although myopathy is the most commonly reported side effect of statin usage, much of it (e.g., myositis) goes undetected. This is because the symptoms are often not accompanied by blood work showing muscle enzyme elevations and can only be detected by biopsies (which are rarely done relative to blood work). In many cases, this condition is permanent (one expert in statin injury found it was permanent for 68% of her patients, while Graveline found it was for 25% of his). Sadly in some cases, like statin neuropathies, the myopathies will continue to progress even if the statin is stopped.
One of the sadder things about statins is how aggressively they are pushed on diabetics (under the logic that since diabetics have an increased risk of heart disease, it is critical a statin to prevent this from happening). To highlight the absurdity of this, a significant side effect of statins is them significantly increasing your risk of diabetes (multiple studies have found this), which I suspect is due to them impairing mitochondrial function.
Similarly, peripheral neuropathy is a condition diabetics are well known to be at a high risk of. In one study, it was found that the risk of neuropathy (i.e., burning pain with tingling or numbness of the extremities) was increased by 14 to 26 times (depending on the type) for long-term users of statins. Furthermore, other nerve issues, such as neurodegeneration, can be caused by statins.
Combinations of myopathy and neuropathy also occur in statin users, such as progressive pain, weakness, and incoordination throughout the body, alongside trouble rising from a seated position, unsteadiness and a tendency to fall. Muscles are also observed to develop a weakened and mushy characteristic and gradually shrink.
Dolichol
Very few physicians know of the dolichols, which play a pivotal role in synthesizing proteins, and Graveline argues, neuropeptides throughout the body. Since neuropeptides are pivotal in your thoughts, emotions, and sensations, statins blocking their production can lead to significant issues. Conversely, trials of dolichols for individuals with dolichol deficiencies have suggested their supplementation safely improves neurodegenerative conditions such as Alzhimers’s disease.
Graveline in turn argues that inhibition of dolichol production and therefore neuropeptide production appears to account for the aggression, hostility, irritability, road rage, homicidal ideation, exacerbation of alcohol and drug addiction, depression, and suicides that are associated with statin use. These side effects are one of the sadder complications of statins I observe in families affected by them.
Note: varying degrees of mood changes and psychoses are also beginning to be associated with the COVID vaccines, which some have theorized explains why certain vaccinated individuals have such blind faith in the products.
Tau Protein
Many neurological disorders (e.g., Parkinson's, Alzheimer's, ALS, MS) are thought to result from misfolded proteins. Because statins interfere with mevalonate synthesis, Graveline theorized that the production of Tau protein would be altered, which provides a potential explanation for the neurological diseases associated with statin usage. I briefly researched this theory when writing this article, and I am unsure if the existing evidence supports it.
Note: There is a strong association between the COVID-19 vaccine and misfolded proteins in the body.
Seleno-protein
To quote Glaveline:
Deficiency of selenoproteins has been proven to result in various types of myopathies formerly seen only in areas known to be deficient in this trace element. Additionally cognitive dysfunction is known to be associated with selenium deficiency.
Note: selenium deficiency is also associated with other diseases such as impaired immune function.
Nuclear Factor-Kappa B
The small cardiovascular benefit observed from statins appears not to be because it reduces cholesterol but because it has anti-inflammatory properties (inflammation causes heart disease) as it inhibits a vital part of the immune system, NF-kB.
Since it suppresses the immune system, this leads to various potential issues, such as reduced protection from infectious disease. For example, many common infectious organisms target NF-kB to assist in infecting their host. However, the more significant issue is that Nf-kB inhibition appears to be linked to cancer.
At five hospitals in Tokyo a group of Japanese researchers studied whether cancer patients had been treated with statins more often than other people. To that end they selected patients with various forms of lymphoid cancers and control individuals of the same age and sex without cancer admitted to other departments at the same hospitals during the same period. A total of 13.3 percent of the cancer patients, but only 7.3 percent of the control individuals were or had been on statin treatment.
In PROSPER [a major statin trial], men and women aged 70-82 were included only. All of them had either vascular disease or had a raised risk of such disease. At follow-up, 4.2 percent had died from a heart attack in the control group, but only 3.3 percent in the treatment group. This small benefit was neutralized by a higher risk of dying from cancer. Indeed, there were 28 fewer deaths from heart disease in the pravastatin group, but 24 more deaths from cancer. If we include non-fatal cancer in the calculation, the cancer difference between the two groups became statistically significant; 199 in the control group and 245 in the pravastatin group. Furthermore the difference between the two groups increased year for year.
In addition to this arguing that some of the benefit of statins “preventing heart attacks” is due to them causing a fatal cancer before you have time to have a heart attack, this situation is somewhat analogous to what was seen with the COVID vaccines (which also cause cancer). There, the “benefit” of the COVID vaccines preventing COVID was outweighed by them causing serious conditions such as heart attacks and strokes, but if one only focused on them preventing COVID (which many did), the vaccines could be portrayed as life-saving, even though they overall did the opposite.
Note: although statins appear to increase cancer, one of the few benefits I have seen a lot of evidence for is their prevention of fatal prostate cancer. My best guess is that this is due to them blocking the production of hormones in the body, and that outweighing the effects of them inhibiting NF-kB.
Conclusion
For over ten years, I have asked holistically-minded physicians I meet what they consider the five most useless, harmful, and overprescribed drugs on the market. Without fail, statins always make their list. Despite this, business continues as usual with statins (which have now been on the market for 35 years), and most doctors without an integrative background have almost no knowledge of anything within this article.
Like many other drug injuries, statin injuries are quite difficult to treat, and after realizing no conventional doctor could help him, Dr. Graveline turned to holistic medicine. Despite effectively being the "expert" on statin injury, he struggled greatly to find an option that could work and settled on a mix of mitochondrial supplements, which facilitated a very gradual improvement for him. His experience is not unique, and as the vaccine injured have found, it is very challenging to find doctors who can treat pharmaceutical injuries, which I believe should serve as a reminder to our profession that we must be far more careful in our prescribing. Consider for a moment the scale of the statin damage crisis, and the fact that decades later there is still almost no one treating it.
Note: one interesting thing Graveline shared is that Earthing was one of the few therapies that helped him. Earthing is one way to (partially) improve zeta potential, and numerous COVID vaccine-injured individuals (a disease I believe partially results from zeta potential disruptions) have reported partial improvement from initiating Earthing.
Since "cholesterol" is ultimately all about money and many statins are beginning to go off-patent to maintain the lucrative cholesterol market, even more dangerous (but patentable) methods are being looked at lower cholesterol. These include the PCSK9 inhibitors, which work by causing cells to overload themselves by sucking cholesterol up from the bloodstream and have a variety of issues, such as causing diabetes. To this point, Pfizer gave up on its PCSK9 inhibitors after their clinical trial showed it killed more participants than the placebo.
Presently two FDA-approved (and extremely expensive) PCSK9 inhibitors are on the market (Repatha and Praluent). A vaccine that causes your immune system to destroy your PCSK9 receptors has also been developed (which strikes me as a terrible idea). Most recently, I learned from a colleague that drug reps visited his office promoting a newly FDA approved RNA therapy (inclisiran) that silences the gene that produces PCSK9 (this also strikes me as a terrible idea).
Statins like vaccines fit remarkably well into the mythology of our culture, where it is believed health can result from taking pills rather than taking responsibility for one's well-being. Brave individuals such as Malcolm Kendrick have done a remarkable job of laying out the core causes of heart disease (which is essentially arterial damage leading to pathologic blood clots when those injuries are repaired and excessive stress). I, too have tried to share (in this article) the additional details I believe Kendrick's excellent model is missing.
Note: Kendrick's model (and its relation to the causes of Alzheimer's disease, such as the spike protein, was discussed further here).
When you look at the actual causes of heart disease, they make a lot of sense and provide a far more consistent explanation than the misguided cholesterol hypothesis we have spent trillions of dollars and decades of national policy on but have nothing to show for. Despite the mountain we face however, there are two reasons why I am quite hopeful this paradigm may change soon.
The first, as Malhotra's interview highlights is that the general public is at their wit's end with the current pharmaceutical paradigm where unjustifiable pharmaceuticals are pushed on the market and we are expected to all take them because the authorities say so. The second reason is that, provided you understand the actual causes of heart disease, the spike protein is one of the most effective agents for causing it. Since there is an immense need to address the tsunami of vaccine injuries we are facing (e.g., heart disease), I believe that will lead many to begin seriously examining Kendrick's model as a statin will not do anything to repair the vascular damage the vaccines have caused.
This article was a bit on the long end. However, I felt I needed to comprehensively address the topic as so many people I know have been injured by statins, and some of the information here is quite difficult to come across. I thank you as always for your kind support which makes this Substack possible, and your sharing it with people who can benefit it.


Great, as usual, but there is a correction to make. Statins do not make 1 trillion a year. It was an estimate, in 2014 by Ioannidis, that total cumulative sales would approach 1 trillion by 2020. But annual sales are around 15 billion (still a great business though) Source : https://www.imarcgroup.com/statin-market
Anyone who didn't take the (literally) 3 minutes necessary to look into Pfizer's record of unremitting corruption and crimes was and is a fool.
Experimental injections from a serial felon for an imaginary illness. What a wild ride and the show continues.
The short list:
In 2008 Pfizer announced that it was setting aside $894 million to settle the lawsuits that had been filed in connection with Bextra and Celebrex.
In 1996 Pfizer was one of 15 large drug companies that agreed to pay more than $408 million to settle a class action lawsuit charging that they conspired to fix prices charged to independent pharmacies.
In 1999 Pfizer pled guilty to criminal antitrust charges that its former Food Science Group unit took part in two international price-fixing conspiracies—one involving the food preservative sodium erythorbate and the other the flavor enhancer maltol. Pfizer agreed to pay fines totaling $20 million.
In 2002 Pfizer agreed to pay $49 million to settle charges that one of its subsidiaries defrauded the federal Medicaid program by overcharging for its cholesterol-lowering drug Lipitor.
In 2016 the Justice Department announced that Pfizer would pay $784 million to settle allegations that Wyeth underpaid rebates to Medicaid on two of its drugs. Later in 2016 the UK's Competition and Markets Authority fined Pfizer the equivalent of $107 million for charging excessive and unfair prices for an epilepsy drug.
In 2000 the FDA warned Pfizer and Pharmacia, co-marketers of the arthritis drug Celebrex, that the consumer ads they were running for the medication were false and misleading.
Two years later, the FDA ordered Pfizer to stop running a series of magazine ads that the agency said misleadingly suggested that its cholesterol-lowering drug Lipitor was safer than competing products.
In 2003 Pfizer paid $6 million to settle with 19 states that had accused the company of using misleading ads to promote its Zithromax medication for children’s ear infections.
In 2004 Pfizer’s Warner-Lambert subsidiary agreed to pay $430 million to resolve criminal and civil charges that it paid physicians to prescribe its epilepsy drug Neurontin to patients with ailments for which the medication was not approved.
In 2007 Pfizer subsidiary Pharmacia & Upjohn agreed to pay $34.7 million to settle federal charges relating to the illegal marketing of its Genotropin human growth hormone.
In 2009, Pfizer was fined $2.3 billion, then the largest healthcare fraud settlement and the largest criminal fine ever imposed in the United States. Pfizer pled guilty to misbranding the painkiller Bextra with "the intent to defraud or mislead,” promoting the drug to treat acute pain at dosages the FDA had previously deemed dangerously high.
John Kopchinski, a former Pfizer sales representative whose complaint helped bring about the federal investigation, told the New York Times: “The whole culture of Pfizer is driven by sales, and if you didn’t sell drugs illegally, you were not seen as a team player.”
In 2010 Pfizer disclosed that during a six-month period the previous year it had paid $20 million to some 4,500 doctors and other medical professionals for consulting and speaking on the
company’s behalf.
In 2011 Pfizer agreed to pay $14.5 million to resolve federal charges that it illegally marketed its bladder drug Detrol.
In 2012 the U.S. Securities and Exchange Commission announced that it had reached a $45 million settlement with Pfizer to resolve charges that its subsidiaries had bribed overseas doctors and other healthcare professionals.
In 2012, Pfizer paid $1.2 billion to settle claims by nearly 10,000 women for punitive damages for the drug maker’s actions in withholding information about the risk of breast cancer from Prempro.
In 2013, Pfizer agreed to pay $55 million to settle criminal charges for failing to warn patients and doctors about the risks of kidney disease, kidney injury, kidney failure, and acute interstitial nephritis caused by its proton pump inhibitor, Protonix.
In 2013, Pfizer set aside $288 million to settle claims by 2,700 people that its drug, Chantix, caused suicidal thoughts and severe psychological disorders. The FDA determined that Chantix is probably associated with a higher risk of a heart attack.
In 2013 Pfizer reached a $35 million settlement relating to the improper marketing of the kidney transplant drug Rapamune brought by more than 40 state attorneys general.
In 2000 the Washington Post published a major exposé accusing Pfizer of testing a dangerous new antibiotic (in 1996) called Trovan on children in Nigeria without receiving proper consent from their parents. This unapproved clinical trial on 200 Nigerian children with its experimental anti-meningitis drug, Trovafloxacin, caused the death of 11 children from kidney failure and left dozens more disabled.
In 2001 Pfizer was sued in U.S. federal court by thirty Nigerian families, who accused the company of using their children as human guinea pigs.
In 2006 a panel of Nigerian medical experts concluded that Pfizer had violated international law.
In 2009 the company agreed to pay $75 million to settle some of the lawsuits that had been brought in Nigerian courts.The U.S. case was settled in 2011 for an undisclosed amount.